Both Mounjaro® (tirzepatide) and Trulicity® (dulaglutide) are GLP-1-type drugs indicated for the treatment of type 2 diabetes, but they work in slightly different ways. Find out what sets the two apart and how they can promote weight loss as a part of a clinician-guided metabolic reset.
What is Mounjaro® (tirzepatide)?
Mounjaro® (tirzepatide) is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that was recently approved by the FDA for the treatment of type 2 diabetes. The drug is manufactured by Eli Lilly & Co. and was approved in May 2022.
Mounjaro® works similarly to the other drugs in the GLP-1 receptor agonist family, but it has additional effects that appears to give it a slight edge. It has a dual-action design, mimicking the action of two incretin hormones involved in blood glucose control: Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). This is the first-in-class medicine to act on both of these receptors.
What does this mean for the drug’s efficacy? According to an article published in Diabetes Therapy, “Co-infusion of GLP-1 and GIP has a synergistic effect, resulting in significantly increased insulin response and glucagonostatic (glucagon stabilizing) response, compared with administration of either hormone alone.”
In other words, combining GLP-1 and GIP receptor agonists in a single drug appears to have a greater impact on blood sugar control than GLP-1 used alone.
The recommended starting dosage of Mounjaro® is 2.5 mg and is administered as a once a week subcutaneous injection in a prefilled pen device . After 4 weeks, doses may be increased in 2.5 mg increments, as tolerated, up to a maximum of 15 mg once weekly. Doses are set and monitored by a healthcare provider and may be adjusted to help patients meet their blood sugar, weight loss, and metabolic health goals.
Is Mounjaro® (tirzepatide) approved for weight loss?
Currently, tirzepatide is indicated for use in patients with type 2 diabetes, but it shows promise as a weight-loss drug as well.
Judging from the results of recent weight loss-focused clinical trials, industry experts predict that Lilly will submit tirzepatide (under a different brand name) for FDA approval for use in patients with overweight and obesity in the near future. In doing so, they’d be following in the footsteps of competitor Novo Nordisk, which received approval for its type 2 diabetes drug semaglutide (Ozempic®) as a weight loss drug (Wegovy®) in 2021.
Participants of the recent SURMOUNT-1 clinical trial saw weight loss of 15.0% for those on a 5 mg dose of Mounjaro® 19.5% for those on a 10 mg dose, and 20.9% for those on a 15 mg dose after a 72-week period. This was compared to weight loss of only 3.1% in participants taking a placebo. Researchers also found tirzepatide to be highly effective in reducing hemoglobin A1C levels.
Experts suspect that, like many other GLP-1s, the drug may also reduce the likelihood of cardiovascular events such as heart attack or stroke. A large-scale trial addressing this question is currently underway.
It’s important to note that Mounjaro® doesn’t produce the same effects in isolation: It is meant to be used alongside lifestyle changes, i.e. healthy changes to food, sleep, and exercise.
What is Trulicity® (dulaglutide)?
Trulicity® is the brand name for dulaglutide, one of the earlier GLP-1 medications to be approved by the FDA for patients with type 2 diabetes. It is a once-weekly subcutaneous injectable medication administered in a prefilled autoinjector. Trulicity is one of several GLP-1 medications prescribed by Calibrate clinicians as a part of Calibrate’s Metabolic Reset. Trulicity®, like Mounjaro®, is manufactured by US-based Eli Lilly & Co. Dosages start at 0.75mg with monthly titration up to 4.5mg weekly.
Trulicity® was FDA-approved in 2014 after data from six unique clinical trials—including a total of 3,342 patients—revealed that treatment with dulaglutide “resulted in greater reductions from baseline in A1c levels compared with placebo.”
Is Trulicity® (dulaglutide) approved for weight loss?
While Trulicity® is not a weight loss drug in and of itself, those who take Trulicity® may lose weight, and dulaglutide may be prescribed off-label for weight loss in patients with obesity or overweight.
As with Mounjaro®, clinical trials of Trulicity® concluded that the drug promoted weight loss when used in conjunction with lifestyle changes (i.e. adjustments to food and exercise). A 52-week clinical trial where dulaglutide was given to 1,842 patients with a mean BMI of 34.2 found that all three doses tested resulted in significant reductions in body weight from baseline at the 36‐week mark. The trial evaluated 1.5 mg, 3.0 mg, and 4.5 mg doses of Trulicity®.
Patients with higher baseline BMI given higher doses of dulaglutide experienced the most weight loss. The authors explain, “Patients had a similar mean percentage weight loss across the BMI subgroups, with those escalated to the 4.5 mg dose having an average weight loss of around 5% in the overall study population and in each BMI subgroup.”
How well Trulicity® works for weight loss on an individual basis will depend on individual factors—ranging from your genetics to your health history. The same set of factors will also play a role in how well you tolerate dulaglutide or any other drug in the GLP-1 family. Fortunately, serious adverse events with Trulicity® are rare.
Comparing tirzepatide (Mounjaro®) and dulaglutide (Trulicity®)
The below chart outlines some of the key similarities and differences between once-weekly subcutaneous tirzepatide and dulaglutide.
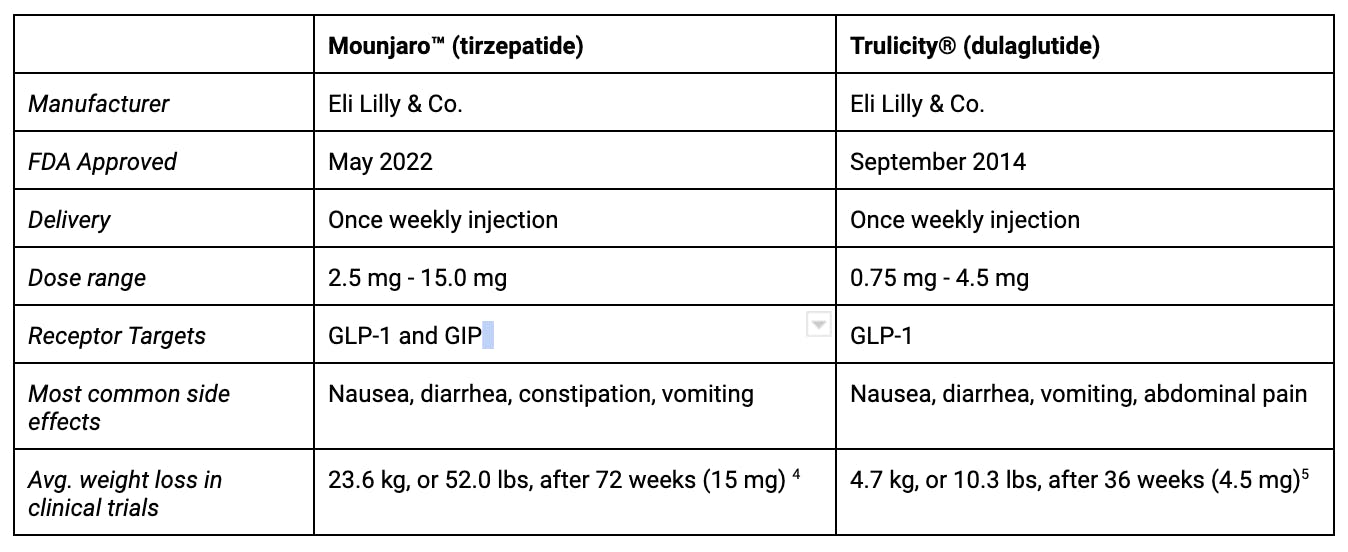
Research thus far points to Mounjaro® (tirzepatide) as the leader in its A1C-reducing and weight loss-promoting effects.
One study, completed in 2018, compared tirzepatide directly to dulaglutide: Results found that tirzepatide “showed significantly better efficacy with regard to glucose control and weight loss than did dulaglutide, with an acceptable safety and tolerability profile.” Side effects with tirzepatide were similar to those of dulaglutide and, in general, the drug was well tolerated.
As mentioned previously, a clinical trial (SURPASS-CVOT) is currently underway that aims to shed light on tirzepatide’s ability to mitigate cardiovascular risk. The SURPASS-CVOT trial is a phase 3, randomized, double-blind cardiovascular outcomes trial that will assess tirzepatide for both “non-inferiority and superiority against dulaglutide.”
For dulaglutide, this effect is already proven: According to the Trulicity® website, the drug may be used in adults with type 2 diabetes “to reduce the risk of major cardiovascular events (problems having to do with the heart and blood vessels) such as death, heart attack, or stroke in people who have heart disease or multiple cardiovascular risk factors.”
How do I know which medication is right for me?
Like all other GLP-1-family drugs, how well Mounjaro® or Trulicity® will work for you will depend on a multitude of factors—including how well you’re able to incorporate lifestyle changes like exercise, healthy food choices, and better sleep hygiene.
Calibrate’s Metabolic Reset is uniquely designed to help members see the best possible results from their GLP-1 medication.
Calibrate uses proven methods for long-term results—the latest science, structured curriculum, FDA-approved medication, one-on-one clinical visits, and accountability coaching—for weight loss of 15% or more. The Calibrate program was designed by world-renowned obesity experts and is vetted and approved by a Clinical Advisory Board.
With Calibrate, members benefit from comprehensive, one-on-one support and coaching that spans four key areas: Food intake, exercise, sleep, and emotional health. These are what we call our Four Pillars of Metabolic Health.
Throughout your Calibrate program, you’ll apply these pillars by making small changes to your daily routine. Small, sustained lifestyle changes combined with GLP-1 medication, a support team, and a science-backed curriculum all come together to make metabolic health achievable.
The GLP-1 medications currently available to Calibrate members include Mounjaro®, Wegovy®, Ozempic®, Rybelsus®, Saxenda®, and Trulicity®. Calibrate clinicians will decide which medication to prescribe based on your unique needs and health insurance coverage.
When you join Calibrate, you’ll complete a Comprehensive Health Intake, including blood work, that your Calibrate clinician will carefully review before customizing your treatment plan and prescribing your medication. You may be eligible for tirzepatide or dulaglutide even if you are already taking another type 2 diabetes medication such as metformin.
Want to learn more about GLP-1s prescribed as a part of the Calibrate program? Read about medications.
Ready to get started with Calibrate? Find out if you’re eligible today.
Sources:
- https://link.springer.com/article/10.1007/s13300-020-00981-0
- https://www.endocrinologynetwork.com/view/surmount-1-tirzepatide-provides-significant-sustained-weight-loss-in-obesity
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8518850/
- https://www.nejm.org/doi/full/10.1056/NEJMoa2206038
- https://diabetesjournals.org/care/article/44/3/765/138650/Efficacy-and-Safety-of-Dulaglutide-3-0-mg-and-4-5
- https://pubmed.ncbi.nlm.nih.gov/30293770/
- https://link.springer.com/article/10.1007/s13300-020-00981-0
- https://www.trulicity.com/
We’re a modern, medical approach that combines clinician-prescribed medication with 1:1 accountability coaching—all personalized to your biology, your goals, and your life for a metabolic reset that lasts and 10% Weight Loss Guaranteed (see terms).
See All from Calibrate